What Acetylide Anion and Alkyl Chloride Can Be Used to Prepare the Following Alkyne?
Acetylide refers to chemical compounds with the chemic formulas MC≡CH and MC≡CM, where Yard is a metal.[ane] The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM (where R is an organic side chain). Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
Structure and bonding [edit]
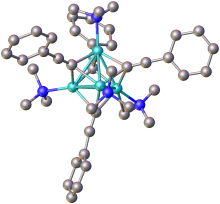
structure of the cluster formed from PhC2Li complexed to Due north,N,N′,N′-tetramethyl-1,6-diaminohexane (methylene groups omitted for clarity). Colour primal: turquoise = Li, blue = Due north.[2]
Alkali metal and alkaline earth metal acetylides of the general formula MC≡CM are table salt-similar Zintl phase compounds, containing C 2−
two ions. Evidence for this ionic graphic symbol can be seen in the fix hydrolysis of these compounds to grade acetylene and metal oxides, in that location is besides some show for the solubility of C two−
ii ions in liquid ammonia.[three] The C 2−
2 ion has a closed shell ground state of 1Σ +
yard , making it isoelectronic to a neutral molecule Due north2,[4] which may afford it some stability.
Analogous acetylides prepared from other metals, especially transition metals, show covalent graphic symbol and are invariably associated with their metal centers. This can be seen in their full general stability to h2o (such every bit silver acetylide, copper acetylide) and radically different chemic applications.
Acetylides of the general formula RC≡CM (where R = H or alkyl) generally prove similar properties to their doubly substituted analogues. In the absence of additional ligands, metal acetylides adopt polymeric structures wherein the acetylide groups are bridging ligands.
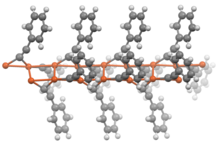
Portion of the construction of the polymer copper phenylacetylide (CuCtwoChalf-dozenH5).[5]
Preparation [edit]
Terminal alkynes are weak acids:[vi]
- RC≡CH + R″Thou ⇌ R″H + RC≡CM
To generate acetylides from acetylene and alkynes relies on the use of organometallic[7] or inorganic[viii] superbases in solvents which are less acidic than the terminal alkyne. In early studies liquid ammonia was employed, but ethereal solvents are more than common.
Lithium amide,[half-dozen] LiHMDS,[9] or organolithium reagents, such every bit butyllithium,[7] are frequently used to grade lithium acetylides:
Sodium or potassium acetylides tin be prepared from diverse inorganic reagents (such as sodium amide)[8] or from their elemental metals, often at room temperature and atmospheric pressure.[half dozen]
Copper(I) acetylide can be prepared by passing acetylene through an aqueous solution of copper(I) chloride because of a low solubility equilibrium.[6] Similarly, silverish acetylides tin can be obtained from silvery nitrate.
Calcium carbide is prepared past heating carbon with lime (calcium oxide) at approximately 2,000 °C. A like process is used to produce lithium carbide.
Reactions [edit]
Acetylides of the type RC2M are widely used in alkynylations in organic chemistry. They are nucleophiles that add to a diversity of electrophilic and unsaturated substrates. A archetype application is the Favorskii reaction.
Illustrative is the sequence shown below, ethyl propiolate is deprotonated by n-butyllithium to give the corresponding acetylide. This acetylide adds to the carbonyl center of cyclopentanone. Hydrolytic workup liberate the alkynyl alcohol.[10]

Coupling reactions [edit]
Acetylides are sometimes intermediates in coupling reactions. Examples include Sonogashira coupling, Cadiot-Chodkiewicz coupling, Glaser coupling and Eglinton coupling.
Hazards [edit]
Some acetylides are notoriously explosive.[eleven] Formation of acetylides poses a risk in handling of gaseous acetylene in presence of metals such as mercury, silver or copper, or alloys with their high content (brass, bronze, silverish solder).
Come across too [edit]
- Ethynyl
- Ethynyl radical
- Diatomic carbon (neutral C2)
- Acetylenediol
References [edit]
- ^ IUPAC, Compendium of Chemic Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acetylides". doi:10.1351/goldbook.A00067
- ^ Schubert, Bernd; Weiss, Erwin (1983). "(PHCCLi)four(tmhda)2, A Polymeric Organolithium Compound with Cubic Li4C4 Structural Units". Angewandte Chemie International Edition in English language. 22 (six): 496–497. doi:x.1002/anie.198304961.
- ^ Hamberger, Markus; Liebig, Stefan; Friedrich, Ute; Korber, Nikolaus; Ruschewitz, Uwe (21 December 2012). "Prove of Solubility of the Acetylide Ion C 2−
2 : Syntheses and Crystal Structures of KiiCtwo·2 NH3, Rb2C2·2 NHiii, and CstwoC2·7 NH3". Angewandte Chemie International Edition. 51 (52): 13006–13010. doi:ten.1002/anie.201206349. PMID 23161511. - ^ Sommerfeld, T.; Riss, U.; Meyer, H.-D.; Cederbaum, L. (August 1997). "Metastable C 2−
2 Dianion". Physical Review Messages. 79 (7): 1237–1240. Bibcode:1997PhRvL..79.1237S. doi:x.1103/PhysRevLett.79.1237. - ^ Chui, Stephen Due south. Y.; Ng, Miro F. Y.; Che, Chi-Ming (2005). "Structure Conclusion of Homoleptic AuI, AgI, and CuI Aryl/Alkylethynyl Coordination Polymers by X-ray Pulverization Diffraction". Chemical science: A European Journal. 11 (6): 1739–1749. doi:10.1002/chem.200400881. PMID 15669067.
- ^ a b c d Viehe, Heinz Günter (1969). "Chemistry of Acetylenes". Angewandte Chemie (1st ed.). New York: Marcel Dekker. 84 (8): 170–179 & 225–241. doi:ten.1002/ange.19720840843.
- ^ a b Midland, M. One thousand.; McLoughlin, J. I.; Werley, Ralph T., Jr. (1990). "Preparation and Use of Lithium Acetylide: 1-Methyl-two-ethynyl-endo-3,3-dimethyl-ii-norbornanol". Organic Syntheses. 68: fourteen. doi:10.15227/orgsyn.068.0014.
- ^ a b Coffman, Donald D. (1940). "Dimethylethhynylcarbinol". Organic Syntheses. 40: xx. doi:10.15227/orgsyn.020.0040.
- ^ Reich, Melanie (August 24, 2001). "Add-on of a lithium acetylide to an aldehyde; 1-(2-pentyn-four-ol)-cyclopent-ii-en-1-ol". ChemSpider Synthetic Pages (Data Set up): 137. doi:10.1039/SP137.
- ^ Midland, M. Mark; Tramontano, Alfonso; Cable, John R. (1980). "Synthesis of alkyl 4-hydroxy-2-alkynoates". The Journal of Organic Chemical science. 45 (ane): 28–29. doi:10.1021/jo01289a006.
- ^ Cataldo, Franco; Casari, Carlo S. (2007). "Synthesis, Structure and Thermal Properties of Copper and Silver Polyynides and Acetylides". Periodical of Inorganic and Organometallic Polymers and Materials. 17 (iv): 641–651. doi:10.1007/s10904-007-9150-3. ISSN 1574-1443. S2CID 96278932.
Source: https://en.wikipedia.org/wiki/Acetylide
![{\displaystyle {\ce {{H-C{\equiv }C-H}+{\overset {butyllithium}{BuLi}}->[{\ce {THF}}][-78^{\circ }{\ce {C}}]{Li-\!{\equiv }\!-H}+BuH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7f2a31939e76759509d17aec8c45e1c46780193c)
0 Response to "What Acetylide Anion and Alkyl Chloride Can Be Used to Prepare the Following Alkyne?"
Post a Comment